Production of gas from methane hydrate
Dissociation of methane hydrate
In order to produce energy from methane hydrate, it is necessary to extract methane (the main component of natural gas) enclosed in the crystalline methane hydrate. However, unlike conventional gas or oil production, this process is not simple because methane exists as a solid in the formation (methane hydrate reservoir).
In the case of conventional natural gas or oil reservoirs where the pressure is sufficiently high and hydrocarbon exists in gas or liquid form, gas /oil normally flows into a well then moves up to the surface under its own pressure once a well is drilled. Methane hydrate, on the other hand, is present as a solid, which has no fluidity, thus cannot be recovered by simply drilling a well. As a result, several methods are being examined to dissociate methane hydrate into gas and water in situ so that it can be extracted through drilled wells. Given those methods, almost the same technologies, equipment, and facilities as those used for conventional natural gas production could be applied.
Methane hydrate, which is stable under low temperature and high-pressure conditions, could leave the stable condition (methane hydrate stability zone) by increasing the temperature or reducing the pressure, thus allowing it to be dissociated into gas and water. The method that MH21-S is mainly tackling at present is the so-called “depressurization method”. Compared to the method in which heat in the form of, for example, hot water is injected underground to raise the temperature, this depressurization method requires simple facilities and is considered superior in terms of energy efficiency.
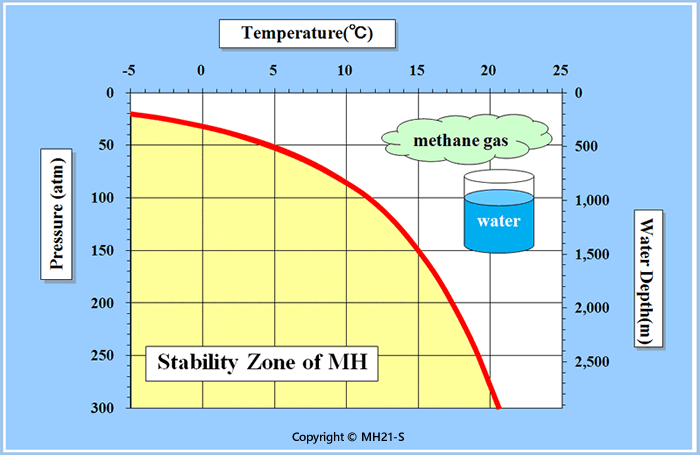
Fig.1 Methane hydrate stability zone curve
The yellow zone represents the area to allow methane hydrate to occur under stable conditions, out of which it dissociates into methane gas and water.
Mechanism of the Depressurization method
This method reduces the pressure inside a well, leading to a reduction in the pressure acting on the formation containing methane hydrate (reservoir pressure), thus promoting the dissociation of methane hydrate in the reservoir and production of methane gas.
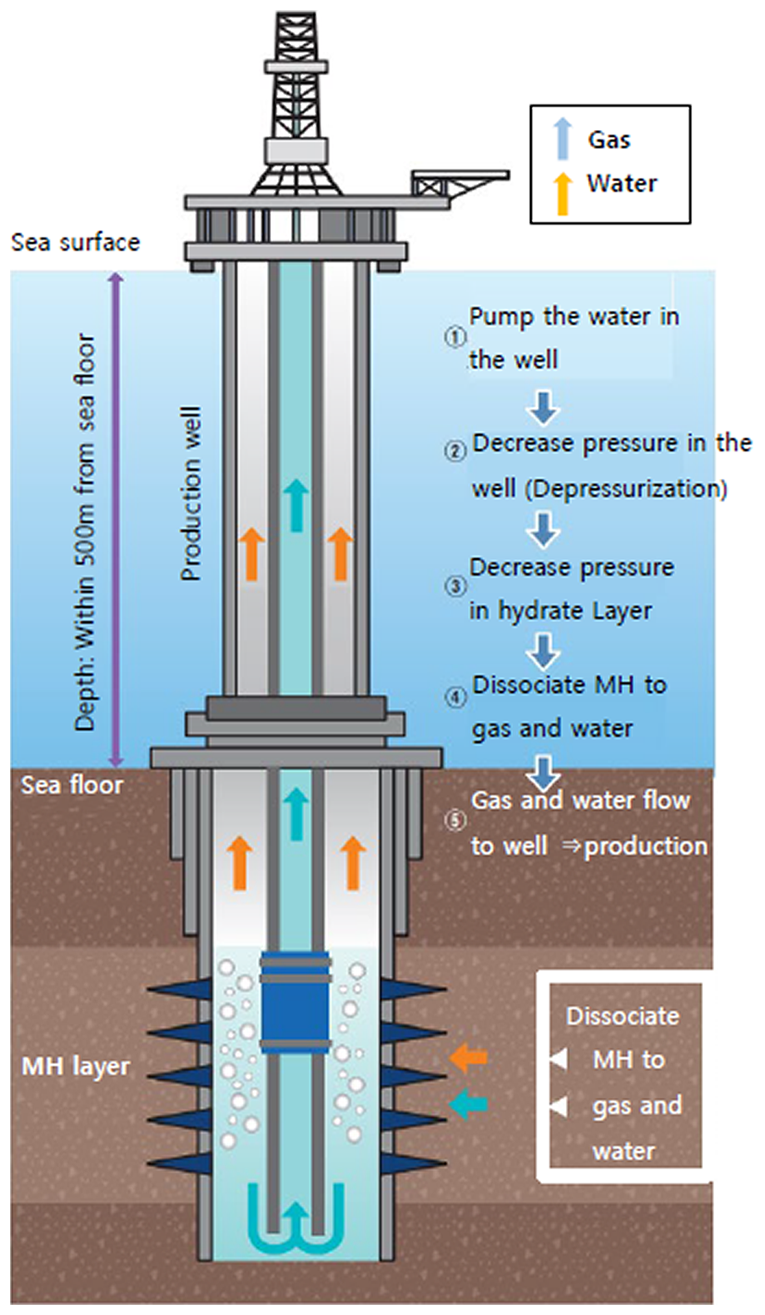
Fig.2 Image of depressurization method
Fig. 2 represents an example of methane hydrate occurring under the seabed. Pressure (hydraulic head pressure) corresponding to that of the water depth plus the depth from the seabed down to the formation containing methane hydrate (methane hydrate reservoir) is applied to the methane hydrate reservoir. In this depressurization method, pumps are used to draw water in the wellbore and reduce the hydraulic head. This reduces the pressure acting on the reservoir to allow methane hydrate around the well to dissociate into methane gas and water. It is considered that changes in pressure (depressurization) expand gradually to areas away from the well, thus the region of dissociation could be enlarged. The methane hydrate reservoirs off the coast of Japan examined so far by MH21-S are sand layers in “sand-mud alternate layers”, where sand and mud layers are laid on top of each other consecutively. Like conventional oil or gas reservoirs, these sand layers have high permeability (ability to allow fluid to pass through) and are believed to have relatively good continuity in the horizontal direction. For these reasons, it is considered that these layers facilitate transmission of pressure changes and also allow water and methane gas, which come out by dissociation, to flow easily.
On the other hand, mud layers above and below sand layers have low permeability, which could confine pressure changes within the sand layers, as well as prevent the intrusion of seawater from the seabed and the upward leakage of methane gas.
Mud layers have another important role. Since the dissociation reaction of methane hydrate is an endothermic process, the temperature of the dissociation area falls as the reaction progresses. At the same time, the heat of the surrounding area is absorbed, therefore, it is thought that the heat supply from mud layers to sand layers could contribute to accelerating dissociation and producing more methane gas.
Consequently, sand layers sandwiched between mud layers are considered to be favorable for the depressurization method.
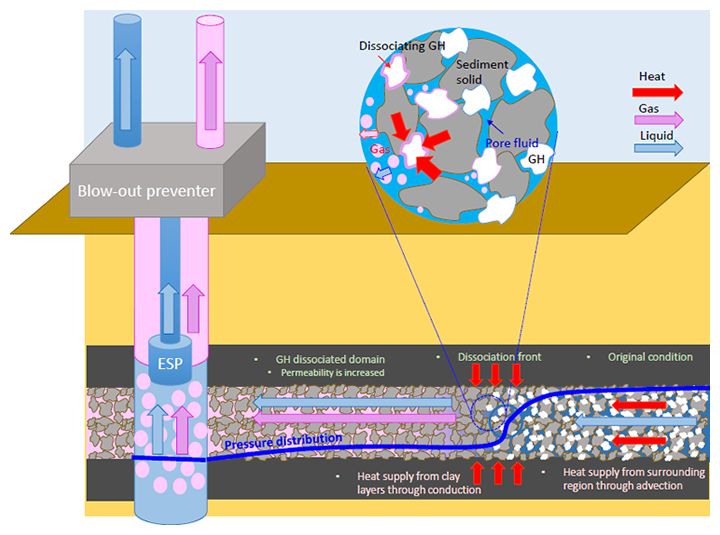
Fig.3 Image of gas production from methane hydrate layers by means of depressurization method employed in the second offshore production test
As the dissociation of methane hydrate is an endothermic reaction that consumes heat, depressurization will gradually reduce the temperature of nearby layers and subsequently make dissociation difficult. Therefore, in order to produce methane gas continuously using the depressurization method, the method of effectively using the heat supplied from the adjacent mud layers and transported water is crucial, and the amount of methane gas to be produced (production rate) depends on how much heat can be supplied.
Merit of depressurization method
The depressurization method has another significant merit, that is, it can apply the knowledge, techniques and equipment so far accumulated for conventional resource development. The technique to extract resources from permeable sand layers has already been developed for use in conventional oil and gas development. The reservoir depth of conventional oil and gas fields is generally much deeper than that of methane hydrate, and the initial reservoir pressure is sufficiently high, therefore, oil/gas can usually be recovered without using any equipment to boost the pressure during the initial production stage. The reservoir pressure, however, gradually falls with production time, causing a decline in production when the reservoir pressure falls below a certain pressure. In such cases, the “artificial lift” technique in which pumps are often installed in the wells is employed in order to maintain or recover production. Such a technique may also be applied to the depressurization method as it is or with some modifications. In actuality, pumps, equipment, and well configuration for conventional resource development were used in past offshore methane hydrate production tests, with modifications/improvements made in certain cases. As a result, the depressurization method needs very little if any equipment to be developed from scratch, which can enable a substantial reduction in development costs and be a big advantage.
Thermal simulation method
As mentioned before, besides depressurization, there is another method of dissociating methane hydrate: thermal stimulation. Even at a constant pressure, increasing temperature will cause deviation from the methane hydrate stability zone in order to initiate dissociation of methane hydrate, as shown in Fig.1. The simplest idea for thermal stimulation is to pour hot water into the well. In fact, production tests carried out in the past employed this method to dissociate methane hydrate. However, this method requires a significant amount of energy to produce hot water, therefore reducing energy efficiency, while the cost of equipment and operation is high. It is thus considered that this thermal stimulation is inferior to the depressurization method from an economic standpoint at present.

