Methane Hydrate
Some key points about the characteristics of methane hydrate
Methane hydrate (known as MH) is a composition of methane and water that is formed under certain temperature and pressure conditions. The vast abundance of this material is thought to exist in the earth’s crust and is considered to contribute to the global carbon cycle, to climate change, and also to large-scale submarine landslides. In the petroleum industry, naturally occurring MH has been known to be a causative effect of geo-hazards that threaten deep-water marine drilling operations. On the other hand, this substance is expected to become an alternative hydrocarbon resource that has the capacity to replace conventional natural gas. Under ambient conditions (with pressure at 100kPa), MH can begin to form when the temperature falls to −80℃ or less. Alternatively, when the pressure approaches 2.6 MPa, MH can begin to form when the temperature reaches 0℃. In either case, methane should supersaturate the water. The latent heat of MH dissociation is 436kJ/kg. This is a value that is 1.3 times larger than that of ice. It is also larger than the energy required to heat the same mass of water to a temperature just below boiling point. The implications of this are, firstly, that dissociation of MH is endothermic, and, secondly, that a large amount of energy is required to exist in order to dissociate MH into its constituent parts of liquid water and vapor methane. The phase diagram of the methane-water system is shown in Fig.1. The phase equilibrium curve of MH and vapor phase methane vary with solute concentration in the water. When the phase equilibrium curve moves mainly in the destabilizing direction, the solute is known as a gas hydrate inhibitor (thermodynamic inhibitor). A material with high solubility and low molecular mass is known as an effective gas hydrate inhibitor. A material that delays association of gas hydrate is known as a kinetic inhibitor. A material that is responsible for the opposite effect (i.e. that accelerates this association) is known as a gas hydrate promotor. Alcohols (ethanol, glycol, etc.) and salts (Sodium Chloride etc.) are used as inhibitors.
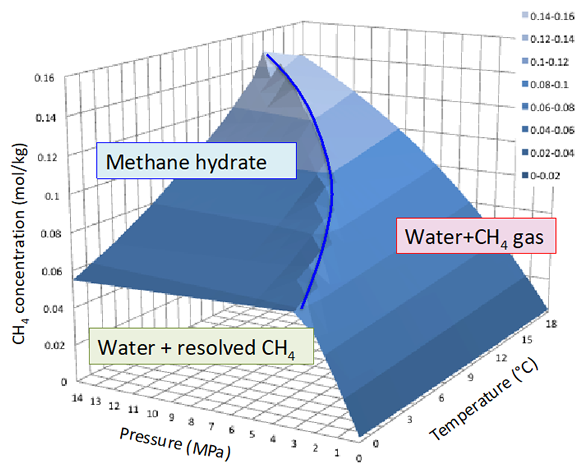
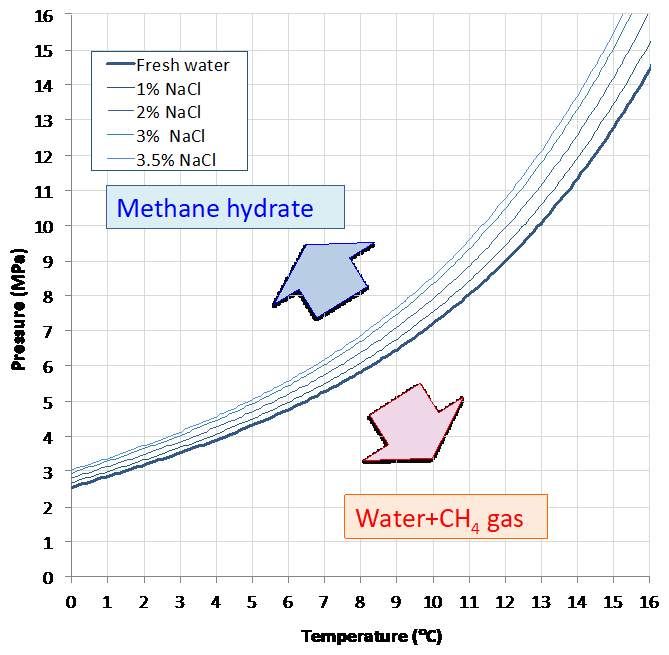
Fig.1 Phase diagrams of methane and water system
On earth, MH is stable in shallow underground deep water regions (i.e. in water depths greater than 400 m and at several hundreds of meters below the sea floor) or beneath permafrost in Arctic areas or else in high altitude mountain regions. Naturally occurring MH is found in several forms, such as the filling in pore spaces in sandy sediments, as a bulk crystal taking the shape of veins or lenses in clayey sediments, or the filling of fractures or faults (Fig.2). In order to gain insights into association and dissociation of MH for the purposes of resource development or in the context of specific environmental and climate conditions, knowledge of the physical properties of the sediment, in particular hydraulic (permeability), thermal (heat capacity and thermal conductivity), and mechanical (strength and elasticities) are as important as an understanding of the properties of MH itself.
Because a sediment containing MH has properties that differ from those containing no MH material, the presence of MH can be detected by use of those geophysical exploration techniques that utilize both elastic waves and electromagnetic methods. The most well-known indicator of MH is the Bottom Simulated Reflector (BSR) by which MH can be detected using seismic exploration. In this case, the lateral extent of MH can be estimated by this reflection.
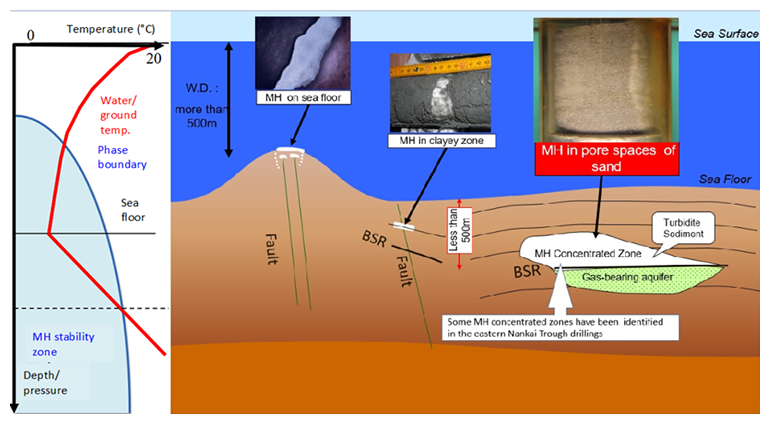
Fig.2 Type of MH with temperature distribution in sea and ground
Many attempts have been made to correctly estimate the quantity of MH residing in the earth’s crust. However, since the estimated value varies with both the process of evaluation and the data used, at this stage it has not been possible to estimate the degree of precision required. On the other hand, uncertainty with respect to this evaluation has been shrinking due to the continued accretion of data built up by global exploration.
In the early stages of this study, it was estimated that 103〜106 Gt of carbon mass (in 1015〜1018 m3 in methane gas volume under ambient conditions) was laid down by pressure and temperature conditions [2]. However, the most recent estimations are in the range of 100〜105 Gt in carbon mass (1014〜1017 m3 in methane gas volume under ambient conditions) . Those estimations only portray the amount of gas in place, and since they do not portray its recovery rate, they cannot be considered to be a true measure of the volume of methane that would be available for use as a resource. Incidentally, the total recoverable volume of natural gas is estimated as being 1014 m3, additionally, world-wide gas consumption annually is estimated to be 4×1012 m3. [US Energy information administration, 2010.]
With regard to the status of this resource on Japanese territory, an estimate of recoverable methane gas was made in 1996. It was based on the BSR area known at the time, and was based on certain assumptions about the vertical distribution of methane (2 m of MH-bearing sediment with 25 % of MH volume fraction and same gas volume of free gas below BSR) and a hypothetical recovery rate (10 % for MH and 75 % for free gas). The value derived for the volume of methane gas under ambient conditions was 2.27×1012 m3. This figure was interpreted in some quarters as being “two-orders of magnitude larger than the annual domestic gas consumption of Japan (5.4×1010 m3 in 1994)”; thereby demonstrating that 100 years of domestic gas consumption could be supplied by MH. However, the value contained a large deal of uncertainty due to the fact that knowledge concerning the occurrence of MH as well as production techniques were at the time rather limited. To put this into perspective, domestic gas consumption in the year 2016 is approximately 1.11×1011 m3. This is almost double that of 1996 and so the “100 years” reference above is highly exaggerated even if the estimation itself is correct.
In 1996, an estimate of recoverable methane gas was made based on the BSR area known at the time, and was based on certain assumptions about the vertical distribution of methane and a hypothetical recovery rate. The value derived for the volume of methane gas under ambient conditions was 2.27×1012 m3 [3] .This figure was interpreted in some quarters as being “two-orders of magnitude larger than the annual domestic gas consumption of Japan (5.4×1010 m3 in 1994).” During the Phase 1 study using seismic survey data and drilling results, MH21 has made a probabilistic estimation of the volume of gas in the eastern Nankai Trough. The median value of this estimate was 1.1×1012 m3. Half of this volume exists in an MH-concentrated zone. This zone contains a high saturation of MH that occurs in substantial intervals in sandy sediment [4][5].
The map below shows where methane hydrates have been extracted directly or were inferred to exist from indirect evidence. There are many red dots shown around Japan because surveys have been carried out from early times in the area.
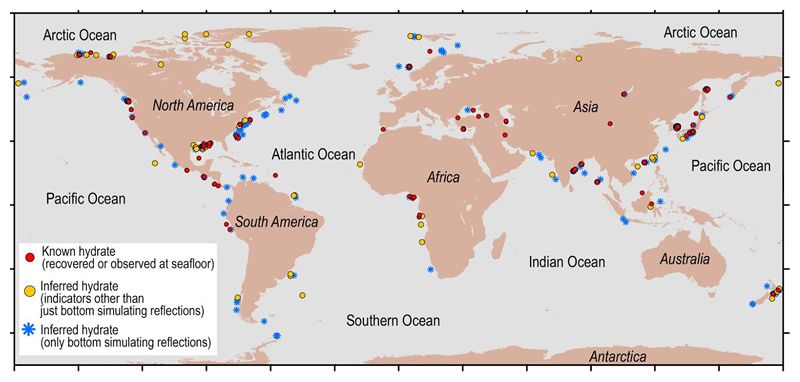
Fig.3 Gas Hydrate locations (known and inferred)
MH21-S added some information around Japan to USGS data
Reference
- [1]Kvebvolden, K.A. (1088): Methane hydrates and global climate, Global Biogeomechemical Cycles, 2(3), 221-229. https://doi.org/10.1029/GB002i003p00221
- [2]Boswell, R. and Collett, T.S. (2011): Current perspectives on gas hydrate resource, Energy Environmental Sci.4, doi: 10.1039/C1030EE00293H, pp.10.
- [3] M. Satoh, T. Maekawa and Y. Okuda (1996): Estimation of amount of methane and resource of natural gas hydrates in the world and around Japan, J. Geol. Soc. Japan, Vol. 102, No.11, 959-971.
- [4] Research Consortium for Methane Hydrate Resources in Japan (2009): Japan’s Methane Hydrate R&D Program Phase 1 Comprehensive Report of Research Results, pp. 15, http://www.mh21japan.gr.jp/english/wp/wp-content/uploads/ca434ff85adf34a4022f54b2503d86e92.pdf
- [5] T. Fujii, T. Saeki, T. Kobayashi, T. Inamori, M. Hayashi, O. Takano, T. Takayama, T. Kawasaki, S. Nagakubo, M. Nakamizu and K. Yokoi (2008): Resource Assessment of Methane Hydrate in the Eastern Nankai Trough, Japan, OTC19310, 2008 Offshore Technology Conference held in Houston, Texas, U.S.A., 5–8 May 2008.

